Vehicle Emissions Trapping Materials

Fig. 1 Configuration of hydrocarbon traps and passive NOx adsorbers for stoichiometric (left) and lean (right) applications.
Overview
The modern three-way catalyst (TWC) is very effective for treating the hydrocarbons (HCs), carbon monoxide (CO), and nitrogen oxides (NOx) from stoichiometric gasoline engines once the TWC has achieved its minimum operating temperature (e.g., 250 to 400 °C, depending on the gas species). Likewise, the diesel oxidation catalyst (DOC), selective catalytic reduction (SCR) catalyst with urea injection, and the diesel particulate filter (DPF) are effective for treating the HCs, CO, NOx, and particulate matter (PM) emissions from diesel engines once the catalysts are warmed up. However, a high portion (up to 80%) of the total vehicle emissions is emitted during the cold start period (i.e., the period before the catalysts are functional).
To treat the HC emissions during this cold start period, one approach is to employ a HC traps (HCTs) that can adsorb the HC emissions at low temperatures and then oxidize the stored HCs to carbon dioxide (CO2) and water (H2O) at higher temperatures. To treat the NOx emissions during the cold start period, a passive NOx adsorber (PNA) can adsorb the NOx at low temperatures (Figure 1). For stoichiometric gasoline applications, the PNA can then reduce the stored NOx to nitrogen (N2) at higher temperatures. On diesel engines, the PNA can release the stored NOx back into the exhaust once the downstream urea/SCR system is operational. Some adsorber technologies have the capability of adsorbing HCs and NOx simultaneously.
This project focuses on the synthesis of ion-exchanged zeolites that have high hydrocarbon and NOx adsorption capacity, high hydrothermal stability and regenerability. The trapping performance of the rationally designed ion-exchanged zeolites is evaluated experimentally under simulated vehicle exhaust conditions. DFT calculations are also used to further elucidate the adsorption capabilities of ion-exchanged zeolites on the molecular level. For example, competitive adsorption of ethylene and toluene is being investigated on Ag/ZSM-5 and H/ZSM-5 at different adsorption sites within the zeolite framework (Figure 2). Our focus is to develop a fundamental understanding of the adsorption/desorption mechanism over mono- and bi-metallic trapping materials.
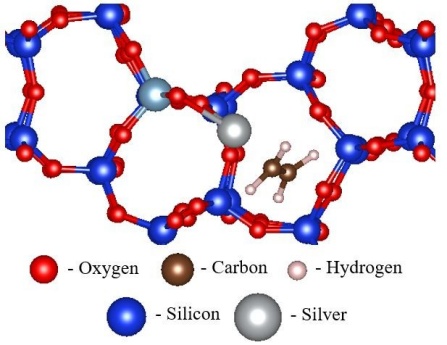
Fig. 2 Physisorption of ethylene within a sinusoidal channel of Ag/ZSM-5
Resulting Publications
- E.A. Kyriakidou, J. Lee, J.-S. Choi, M. Lance, T.J. Toops, “A comparative study of silver- and palladium-exchanged zeolites in propylene and nitrogen oxide adsorption and desorption for cold-start applications.” Catalysis Today, DOI: 10.1016/j.cattod.2020.05.019 (2020).
- C. Horvatits, D. Li, M. Dupuis, E.A. Kyriakidou, E.A. Walker, “Ethylene and Water Co-Adsorption on Ag/SSZ-13 Zeolites: A Theoretical Study.” Journal of Physical Chemistry C, 124, 7295-7306 (2020).
- C. Horvatits, J. Lee, E.A. Kyriakidou, E.A. Walker, “Characterizing Adsorption Sites on Ag/SSZ-13 Zeolites: Experimental Observations and Bayesian Inference.” Journal of Physical Chemistry C, DOI: 10.1021/acs.jpcc.0c06470 (2020).
- J. Lee, J.R. Theis, E.A. Kyriakidou, “Vehicle emissions trapping materials: successes, challenges, and the path forward.” Applied Catalysis B: Environmental, 243, 397-414 (2019).
Students on this Project
- Jungkuk Lee (PhD)
- Kevin Giewont (MS)








































